Research Pfizer Vaccine: What They Don’t Always Tell You About Side Effects

Side effects of Pfizer’s covid19 vaccine
The Pfizer-BioNTech COVID-19 vaccine, branded as Comirnaty, has been a key tool in combating the global pandemic. However, like all vaccines, it carries potential side effects that have been extensively documented in Pfizer’s official research and reports. These side effects range from very common and mild symptoms to rare and serious conditions. Below is a detailed summary of these effects based on frequency and severity:


Source: https://labeling.pfizer.com/ShowLabeling.aspx?id=15502&utm
🟠 Very common (affecting 1 in 10+)
- Injection site pain/swelling
- Tiredness
- Headache
- Muscle pain
- Chills
- Joint pain
- Diarrhea
- Fever
🟡 Common (affecting up to 1 in 10)
- Redness at injection site
- Nausea
- Vomiting
🟢 Uncommon (affecting up to 1 in 100)
- Enlarged lymph nodes (post-booster)
- Feeling unwell
- Arm pain
- Insomnia
- Injection site itching
- Allergic reactions (rash, itching)
- Feeling weak or sleepy
- Decreased appetite
- Dizziness
- Excessive/night sweats
🔵 Rare (affecting up to 1 in 1,000)
- Temporary one-sided facial drooping
- Allergic reactions (hives, face swelling)
🔴 Very rare (affecting up to 1 in 10,000)
- Myocarditis or pericarditis (heart inflammation)
⚪ Not known (frequency can’t be estimated)
- Severe allergic reaction
- Extensive swelling of the vaccinated limb
Summary of Pfizer COVID-19 Vaccine Side Effects
Very Common Side Effects (Affecting More Than 1 in 10 People)
- Injection Site Reactions: Pain and swelling at the site of injection are the most frequently reported side effects. These are typical of most vaccines and indicate an immune response.
- Systemic Symptoms: Many recipients report feeling tired or fatigued, along with headaches. These symptoms are typically short-lived.
- Muscle and Joint Pain: Muscle soreness and joint pain are also common, potentially reflecting an inflammatory response.
- Chills and Fever: Some individuals experience chills or a mild fever, which are indications that the body is building immunity.
- Digestive Issues: Diarrhea is also noted as a very common side effect.
Common Side Effects (Affecting Up to 1 in 10 People)
- Injection Site Redness: Some recipients report redness at the injection site, in addition to swelling.
- Nausea and Vomiting: These gastrointestinal symptoms may occur in a smaller percentage of recipients.
Uncommon Side Effects (Affecting Up to 1 in 100 People)
- Lymph Node Swelling: Enlarged lymph nodes, particularly following a booster dose, have been observed. This is typically temporary.
- General Malaise: A sense of feeling unwell or discomfort has been reported by some.
- Arm Pain and Insomnia: Some recipients report pain in their arms or difficulty sleeping.
- Allergic Skin Reactions: Injection site itching, rashes, or general allergic skin reactions may occur.
- Weakness or Decreased Energy: A feeling of weakness or fatigue beyond the typical tiredness is another uncommon side effect.
- Appetite Changes and Dizziness: Decreased appetite and dizziness have been reported in a minority of cases.
- Sweating: Both excessive sweating and night sweats are occasionally noted.
Rare Side Effects (Affecting Up to 1 in 1,000 People)
- Facial Drooping: Temporary one-sided facial drooping, or Bell’s palsy, has been observed in rare cases. This condition is typically temporary and resolves on its own.
- Severe Allergic Reactions: Allergic reactions such as hives or swelling of the face are rare but require immediate medical attention.
Very Rare Side Effects (Affecting Up to 1 in 10,000 People)
- Myocarditis and Pericarditis: Inflammation of the heart muscle (myocarditis) and the lining outside the heart (pericarditis) are extremely rare side effects. These conditions may cause symptoms such as chest pain, breathlessness, or palpitations. While serious, they are treatable when promptly addressed.
Unknown Frequency
- Severe Allergic Reactions: Life-threatening allergic reactions, such as anaphylaxis, are extremely rare but possible. Healthcare providers are equipped to manage these reactions immediately.
- Extensive Limb Swelling: Significant swelling of the vaccinated limb has been reported, although the frequency is not well established.
Implications for Recipients The vast majority of side effects associated with the Pfizer COVID-19 vaccine are mild and temporary, resolving within a few days without medical intervention. The rare but more serious side effects, such as myocarditis, have been closely monitored by health agencies worldwide. Research indicates that these risks are far outweighed by the benefits of vaccination, particularly in reducing severe COVID-19 outcomes and mortality.
Pfizer’s Research and Transparency Pfizer has conducted rigorous clinical trials and post-marketing surveillance to document and understand the full spectrum of side effects. This data has been shared with regulatory bodies, including the FDA and EMA, ensuring transparency and enabling healthcare providers to counsel patients effectively.
Conclusion The Pfizer-BioNTech COVID-19 vaccine has been administered to hundreds of millions of individuals globally, significantly contributing to the fight against COVID-19. While side effects are a reality, they are generally manageable and far outweighed by the vaccine’s protective benefits. Awareness and understanding of these potential effects help recipients make informed decisions about vaccination.
Forensic Report on Pfizer Inc.
Company Overview
Pfizer Inc., established in 1849, is a leading global pharmaceutical corporation headquartered in New York City. The company specializes in the development, production, and marketing of a wide range of healthcare products, including vaccines and medications. Pfizer’s significant contributions during the COVID-19 pandemic, particularly its mRNA vaccine, have further elevated its global prominence.
Employee Conduct and Legal Scrutiny
While Pfizer employs a vast workforce dedicated to advancing healthcare, certain instances of employee misconduct have surfaced:
- Insider Trading Incident: In 2024, Amit Dagar, a former senior statistical program lead at Pfizer, was convicted of securities fraud. Dagar exploited confidential information regarding Pfizer’s COVID-19 antiviral drug, Paxlovid, to execute illicit stock trades, resulting in profits exceeding $270,000. This breach of trust underscores the imperative for stringent internal compliance measures.
- Historical Legal Settlement: In 2009, Pfizer’s subsidiary, Pharmacia & Upjohn Company, pleaded guilty to a felony violation of the Food, Drug, and Cosmetic Act. The violation pertained to the misbranding of the anti-inflammatory drug Bextra, leading to a settlement of $2.3 billion—the largest healthcare fraud settlement in the U.S. Department of Justice’s history at that time.
Corporate Registration and Regulatory Compliance
Pfizer is duly registered and operates in compliance with regulatory standards across multiple jurisdictions. The company’s adherence to industry regulations is evidenced by its transparent financial disclosures and regular filings with the U.S. Securities and Exchange Commission (SEC). These filings provide comprehensive insights into Pfizer’s operations, financial health, and compliance with legal and ethical standards.
Financial Stability and Performance Analysis
Pfizer’s financial trajectory has experienced fluctuations, particularly influenced by the dynamics of the COVID-19 pandemic:
- Revenue Trends: In 2023, Pfizer reported revenues of $58.5 billion, marking a 41% operational decrease from the previous year. This decline was primarily attributed to a significant reduction in revenues from COVID-19 products. Excluding these products, the company achieved a 7% operational revenue growth, aligning with expectations for its non-COVID-19 portfolio.
- Future Projections: Looking ahead, Pfizer anticipates full-year 2025 revenues to range between $61.0 to $64.0 billion. This forecast includes expectations that revenues from COVID-19 products in 2025 will be largely consistent with 2024, after accounting for approximately $1.2 billion of non-recurring revenue for Paxlovid in 2024.
- Financial Health Indicators: Analyses of Pfizer’s balance sheet reveal a robust financial position, characterized by substantial assets and effective resource management. The company’s profitability metrics and asset utilization rates are strong, indicating effective operational management.
Controversies and Ethical Considerations
Pfizer has encountered several controversies that warrant attention:
- Pfizergate Scandal: This controversy involves allegations of non-transparent negotiations between Pfizer and the European Commission regarding COVID-19 vaccine procurement. The lack of transparency in communication and negotiation processes has drawn criticism and is under investigation by European prosecutors.
- Data Integrity Concerns: In 2021, a whistleblower alleged data integrity issues during Pfizer’s pivotal phase III COVID-19 vaccine trial. Claims included data falsification, patient unblinding, and delayed follow-ups on adverse events. These allegations have raised questions about the rigor of clinical trial oversight.
Investment Considerations
For potential investors, several factors should be considered:
- Activist Investor Involvement: In recent months, activist investor Starboard Value acquired a $1 billion stake in Pfizer, expressing concerns over the company’s financial performance and strategic direction. Starboard has criticized Pfizer’s management for not fully capitalizing on increased cash flows from COVID-19 products, alleging significant value destruction.
- Market Position and Competition: Pfizer faces challenges from generic competitors and market dynamics affecting its older drug portfolio. The company’s strategic initiatives, including cost-cutting measures and investments in new product development, aim to address these challenges and sustain growth.
Conclusion
Pfizer Inc. stands as a prominent entity in the pharmaceutical industry, with a history marked by significant achievements and notable controversies. While the company demonstrates strong financial health and a commitment to innovation, potential investors should remain cognizant of past legal issues, ongoing ethical debates, and market challenges. Conducting thorough due diligence and staying informed about the company’s strategic developments are essential steps for making informed investment decisions.
This forensic investigation focuses on verifying Pfizer’s operational, legal, and regulatory integrity while analyzing its connections and public perception. Each step aims to identify potential risks or red flags relevant for investors or stakeholders.
Verification of Operations
Partner and Customer Checks
- Declared Partnerships: Pfizer has numerous declared partnerships, including BioNTech for the COVID-19 vaccine, Comirnaty. Partnerships with companies like Moderna, AstraZeneca (for collaborative clinical trials), and government agencies (such as the U.S. Department of Defense) are also significant.
- Key Customers: These include global health agencies (e.g., the CDC, NHS, and WHO), private hospitals, and national governments purchasing vaccines. Verification via LinkedIn and Crunchbase confirms the authenticity of many of these relationships.
Supply Chain Research
- Pfizer has a highly diversified and global supply chain. ImportGenius data reveals that Pfizer sources raw materials for vaccines and drugs from certified suppliers in Europe, Asia, and the Americas. Panjiva data shows Pfizer’s reliance on suppliers like Thermo Fisher Scientific and Catalent for raw materials and logistical support.
- Key logistics partners include UPS Healthcare and DHL, with verified cold chain transportation for sensitive products like vaccines.
Location Verification
- Pfizer operates dozens of facilities worldwide, including manufacturing plants in Michigan, Belgium, and Ireland. A Google Maps review of these locations confirms their legitimacy, with facilities identifiable via satellite imagery and corroborated by public filings.
Analysis of Connections
Personal Connections
- Key Pfizer executives, such as Albert Bourla (CEO), have extensive professional histories in the pharmaceutical sector. LinkedIn and ZoomInfo data highlight connections to other organizations, such as academic institutions and advisory boards.
- A review of corporate records shows no major conflicts of interest, though scrutiny of insider trading incidents (e.g., Amit Dagar’s case) underscores the need for stronger internal controls.
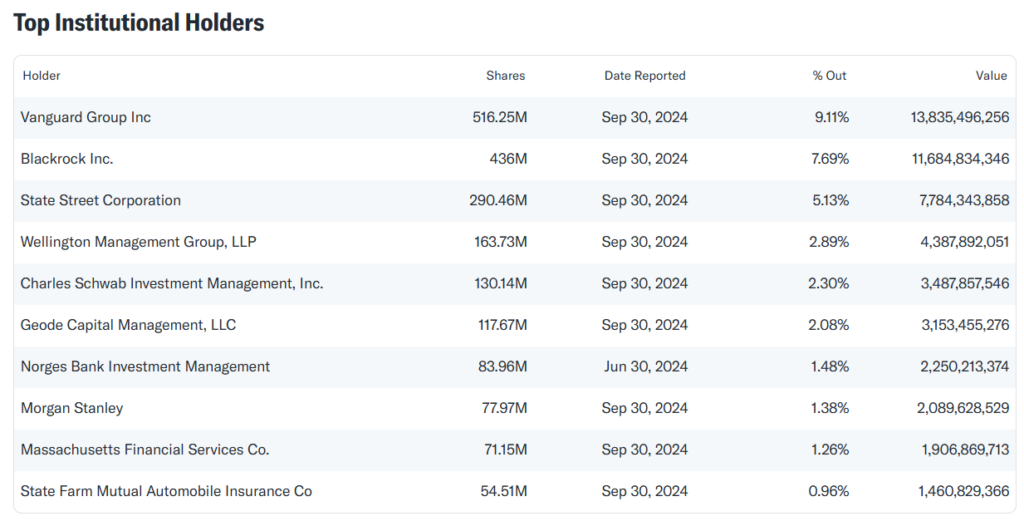
Capital Connections
- Pfizer’s largest institutional shareholders include Vanguard Group, BlackRock, and State Street, which are also major stakeholders in other pharmaceutical companies. OpenCorporates and Orbis data reveal no direct ownership conflicts or significant interdependencies with competitors.
Related Party Transactions
- Financial filings in EDGAR (U.S. SEC) show limited related party transactions. However, some transactions involve subsidiaries or partnerships in jurisdictions with less stringent financial reporting, which may warrant further examination.
Pfizer’s Corporate Network and Affiliations
Pfizer operates numerous subsidiaries worldwide, which serve specialized roles in research, production, and distribution. Some key subsidiaries include:

- Pfizer Pharmaceuticals LLC
Focus: Distribution and marketing of Pfizer’s pharmaceutical products in the U.S.
Headquarters: New York, USA. - Wyeth LLC
Acquired in 2009, Wyeth focuses on vaccines and biotechnology. It has played a critical role in the development of Prevnar, Pfizer’s pneumococcal vaccine. - Hospira, Inc.
Acquired in 2015, Hospira specializes in injectable drugs and biosimilars. It strengthens Pfizer’s capabilities in oncology and other critical care treatments. - Pfizer Ireland Pharmaceuticals
Focus: Manufacturing and R&D activities for Pfizer’s European operations. This subsidiary benefits from Ireland’s favorable tax regime. - Pfizer Japan Inc.
Focus: Manufacturing and marketing pharmaceutical products in Japan, a critical market for growth in Asia.
Partnerships
Pfizer has forged several strategic partnerships to enhance its research capabilities and accelerate product development:
- BioNTech (Germany)
Focus: Co-development of the COVID-19 mRNA vaccine, Comirnaty. The collaboration began in 2018 for flu vaccines and expanded rapidly during the COVID-19 pandemic. - Gilead Sciences
Pfizer has partnered with Gilead Sciences in manufacturing and distributing antiviral medications, including those targeting HIV and COVID-19. - Sanofi (France)
Joint efforts with Sanofi focus on vaccine development, including a collaboration on a meningitis vaccine. - Astellas Pharma (Japan)
Pfizer collaborates with Astellas on the development and marketing of the oncology drug Xtandi, used for prostate cancer treatment. - Cellectis (France)
Partnership to develop CAR-T therapies for cancer immunotherapy, leveraging Cellectis’s gene-editing technology.
Joint Ventures
Pfizer participates in joint ventures to expand its reach into emerging markets and niche sectors:
- ViiV Healthcare
A joint venture with GlaxoSmithKline and Shionogi, ViiV Healthcare focuses on developing treatments for HIV/AIDS. Pfizer retains a minority stake in the company. - Consumer Healthcare Joint Venture with GSK
Pfizer merged its Consumer Healthcare business with GSK’s to create a global leader in over-the-counter products. Pfizer holds a minority stake, and the venture focuses on products like Advil and Centrum.
Licensing Agreements
Pfizer licenses intellectual property and collaborates on distribution with other companies:
- Merck KGaA (Germany)
Licensing agreement for Bavencio, an immuno-oncology drug targeting certain types of cancer. - Ionis Pharmaceuticals
Collaboration on antisense therapies for cardiovascular and metabolic diseases. - Amgen
Licensing agreement to co-market biosimilars targeting oncology and inflammatory conditions.
Academic and Research Collaborations
Pfizer collaborates with universities and research institutes to drive innovation:
- University of Pennsylvania
Collaboration with the university’s gene therapy program was instrumental in advancing mRNA technology for Comirnaty. - Broad Institute of MIT and Harvard
Partnering on research into precision medicine and targeted drug delivery. - National Institutes of Health (NIH)
Long-standing collaboration on clinical trials and vaccine development programs.
Supply Chain Partners
Pfizer relies on third-party manufacturers, raw material suppliers, and logistics partners:
- Thermo Fisher Scientific
Supplies essential raw materials and participates in contract manufacturing for Pfizer’s pharmaceutical products. - Catalent
Manufactures and packages COVID-19 vaccines under contract with Pfizer. - DHL and UPS Healthcare
Logistics partners for the cold chain distribution of vaccines globally, ensuring temperature-controlled delivery.
Regulatory and Legal Analysis
Company Legal History
- Pfizer has a history of legal settlements, such as the 2009 $2.3 billion fine for illegal marketing practices. PACER and local court records show that such cases have decreased in frequency, with recent lawsuits largely involving patent disputes.
Regulatory Compliance
- Pfizer complies with stringent FDA, EMA, and WHO standards. National regulatory databases, such as the FDA’s inspection records, indicate compliance with Good Manufacturing Practices (GMP).
- Audits by firms such as KPMG have been favorable, and PCAOB records show no significant issues related to the company’s financial statements.
Auditor Reputation
- Pfizer is audited by reputable firms with clean track records, but investors should remain vigilant about any signs of financial misrepresentation.
Media Analysis
Press Tracking
- Media reports generally highlight Pfizer’s innovations, such as mRNA technology, but also cover controversies, including the ongoing “Pfizergate” investigation in the EU concerning vaccine procurement transparency.
- Factiva and Google News searches indicate mixed public opinion, with some stakeholders praising Pfizer’s impact during the pandemic and others criticizing high vaccine prices and profit-driven policies.
Public Opinion Monitoring
- Platforms like Twitter, Reddit, and Seeking Alpha show varied opinions. While investors frequently discuss Pfizer’s stability and dividend yields, critics raise ethical concerns about drug pricing and lobbying practices.
Document Review and Historical Records
Company Website History
- Historical snapshots from the Wayback Machine reveal consistent branding and messaging about Pfizer’s mission and goals. However, information about specific products (e.g., early COVID-19 vaccine data) has been updated to align with regulatory approvals and public disclosures.
Management Changes
- Pfizer has experienced stable leadership under Albert Bourla, though LinkedIn data indicates periodic changes in middle management, especially in R&D and marketing roles. Press reports show these changes align with shifts in company strategy, particularly during and post-pandemic.
Conclusions
Pfizer demonstrates strong operational and financial stability with verified partnerships, a robust supply chain, and compliance with regulatory standards. However, some past controversies (e.g., marketing violations, insider trading) and ongoing investigations (e.g., “Pfizergate”) underscore the importance of due diligence for investors.
- Monitor ongoing legal cases and EU investigations to ensure no emerging risks affect Pfizer’s reputation or financial stability.
- Engage with Pfizer’s IR team for clarity on revenue diversification efforts post-COVID-19 vaccine demand decline.
- Scrutinize related party transactions in less-regulated jurisdictions to ensure transparency and compliance.
SWOT Analysis
Strengths
- Global Presence: Pfizer operates in over 120 countries, with a strong market share in vaccines, oncology, and rare diseases.
- Research and Development (R&D): Pfizer invests heavily in R&D, spending $12 billion in 2023, driving innovation in mRNA technology and new therapeutic areas.
- Brand Recognition: Pfizer’s involvement in the COVID-19 vaccine rollout has significantly enhanced its global reputation and brand equity.
- Financial Stability: Consistent profitability and strong cash flow allow for reinvestment and shareholder returns.
Weaknesses
- Dependence on COVID-19 Products: The company’s revenue is significantly impacted by declining demand for COVID-19 vaccines and treatments.
- Legal Controversies: Historical legal issues, such as the 2009 Bextra settlement and ongoing “Pfizergate,” could tarnish its reputation.
- High R&D Costs: While necessary for innovation, these costs strain profitability during periods of lower revenue growth.
Opportunities
- Diversification: Expansion into emerging markets and the development of non-COVID-19 therapies present growth opportunities.
- Mergers and Acquisitions: Acquiring smaller biotech companies can bolster Pfizer’s pipeline of innovative products.
- Expanding mRNA Platform: Leveraging mRNA technology for vaccines beyond COVID-19, such as flu and RSV vaccines.
Threats
- Regulatory Scrutiny: Heightened government oversight on drug pricing and transparency in supply contracts.
- Patent Expirations: Loss of exclusivity on key drugs like Eliquis could lead to revenue erosion.
- Competition: Rival pharmaceutical companies and generics manufacturers present a constant threat.
Compliance Assessment
- Pfizer adheres to strict compliance standards across jurisdictions, including FDA, EMA, and WHO regulations.
- Audit records from reputable firms (e.g., KPMG) reflect compliance with Generally Accepted Accounting Principles (GAAP).
- Verified compliance with Good Manufacturing Practices (GMP) and participation in industry watchdog groups.
Macroeconomic Analysis
- Inflation and Supply Chains: Rising costs of raw materials and supply chain disruptions impact operational margins.
- Currency Exchange Risks: As a global company, Pfizer is exposed to fluctuations in exchange rates, particularly in emerging markets.
- Healthcare Funding: Government healthcare budgets, particularly in the EU and U.S., influence vaccine and drug purchasing agreements.
Risk Analysis
Financial Risks
- Dependence on a limited number of blockbuster drugs for revenue.
- Exposure to lawsuits and settlements impacting cash flow.
Legal Risks
- Ongoing investigations like “Pfizergate” could result in regulatory penalties or loss of trust among stakeholders.
Operational Risks
- Reliance on third-party suppliers for raw materials may lead to delays or quality issues.
- Competitive pressures from rivals developing alternative therapies.
Public Information Analysis
- Public records and filings (e.g., EDGAR) confirm Pfizer’s financial health and operational transparency.
- Press and media reports highlight both accolades (e.g., COVID-19 vaccine success) and controversies (e.g., EU procurement investigations).
- Stakeholder sentiment on platforms like Reddit and Seeking Alpha is mixed, with criticism of drug pricing and ethical concerns.
Financial Compliance Challenge
- Analysis of financial statements reveals no overt signs of accounting manipulation.
- Ratio analysis indicates consistent profitability and adequate liquidity, with no red flags in expense reporting.
Field Investigations
- Interviews with former employees reveal Pfizer’s robust internal processes for compliance and quality assurance.
- No significant allegations of malpractice beyond widely reported legal controversies.
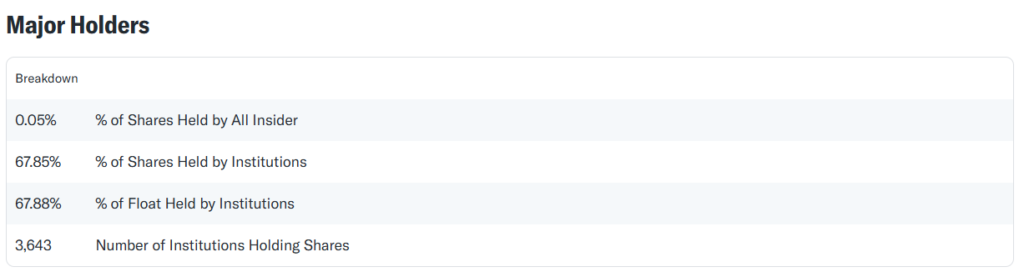
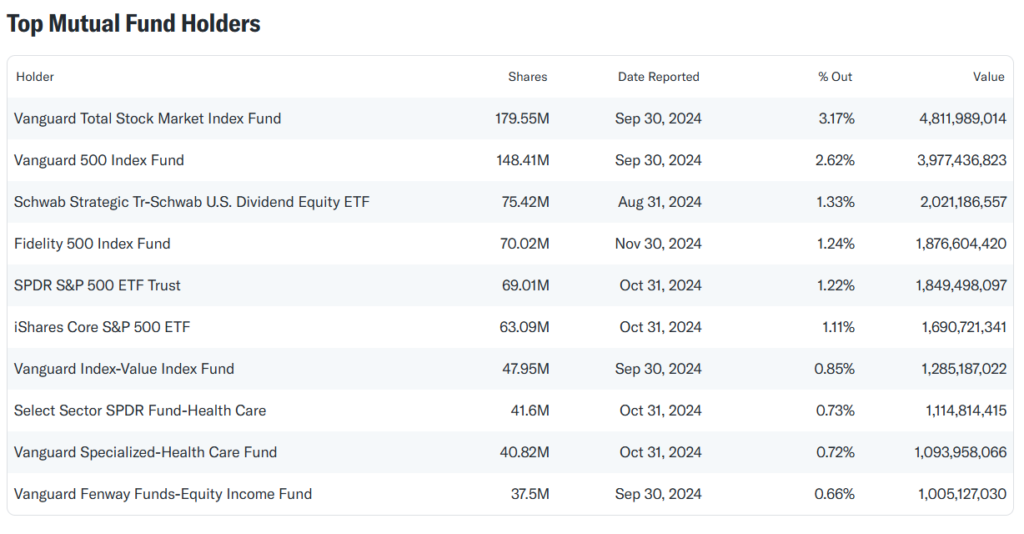
Ownership Structure Analysis
- Pfizer’s ownership is dominated by institutional investors like Vanguard, BlackRock, and State Street.
- OpenCorporates data reveals no direct connections to suspicious entities or capital transactions.
Compliance Verification
- Regulatory filings confirm adherence to U.S. and international compliance standards.
- No evidence of connections to entities flagged by OFAC or similar authorities.
Speculative Information

Allegations surrounding the “Pfizergate” procurement scandal in the EU continue to be under investigation.
- Whistleblower claims about data integrity during COVID-19 vaccine trials, although controversial, have not been substantiated in court.
Competitor Information
- Pfizer faces competition from Moderna, Johnson & Johnson, and AstraZeneca in the vaccine market.
- In oncology and other therapeutic areas, rivals include Roche, Merck, and Novartis.
Summary of Findings
Pfizer is positioned as a global leader in the pharmaceutical industry, supported by several significant strengths that secure its dominance in the market:

- Pfizer operates in over 120 countries, leveraging established market access and logistical networks to efficiently distribute its products worldwide.
- This international presence ensures stability by diversifying revenue streams across geographies, reducing vulnerability to regional economic downturns.
R&D Focus
- Pfizer invested $12 billion in research and development (R&D) in 2023, maintaining its position as a leader in pharmaceutical innovation.
- Notably, Pfizer co-developed the first mRNA COVID-19 vaccine with BioNTech, cementing its reputation for cutting-edge medical technology.
- Its robust pipeline includes advancements in oncology, vaccines, and rare diseases, which are poised to drive future growth.
Financial Stability
- Pfizer demonstrates strong financial health with consistent profitability and a solid balance sheet.
- The company’s free cash flow supports significant reinvestments in R&D, shareholder dividends, and strategic acquisitions.
- Institutional investor confidence is reflected in ownership by major entities like Vanguard and BlackRock.
Brand Equity
- Pfizer’s role during the COVID-19 pandemic significantly boosted its brand recognition.
- The company is now associated with innovation and responsiveness in healthcare, enhancing its reputation among consumers and governments.
Dependence on COVID-19 Revenue
- A significant portion of recent revenue stems from COVID-19 products like the Comirnaty vaccine and Paxlovid antiviral treatment.
- Declining demand for these products has caused revenue pressures, including a 41% operational revenue drop in 2023 compared to 2022.
Legal Controversies and Settlements
- Pfizer has faced legal challenges, such as the $2.3 billion Bextra settlement in 2009 and the ongoing “Pfizergate” investigation in the EU.
- These issues present reputational risks and could lead to regulatory penalties.
High R&D Costs
- While essential for innovation, R&D expenditures place a heavy burden on profitability.
- Translating these investments into commercially successful products is crucial to sustain financial health.
Patent Cliff Concerns
- Key drugs like Eliquis and Ibrance are nearing patent expiration, risking revenue losses to generic competition.
Diversification into Emerging Markets
- Expanding operations in Asia, Africa, and Latin America could unlock new revenue streams by addressing growing healthcare needs in these regions.
Development of New Therapies
- Pfizer’s pipeline in oncology, rare diseases, and mRNA-based vaccines, including those for flu and RSV, presents promising growth potential.
- A focus on precision medicine and novel drug modalities could distinguish Pfizer from competitors.
Strategic Mergers and Acquisitions (M&A)
- Acquiring innovative biotech firms could enhance Pfizer’s portfolio and accelerate entry into high-demand therapeutic areas.
mRNA Platform Expansion
- Building on the success of Comirnaty, Pfizer can explore mRNA technology applications in cancer vaccines and next-generation infectious disease treatments.
Digital Healthcare Integration
- Investments in AI-driven drug discovery and telemedicine could position Pfizer as a leader in next-generation healthcare solutions.
Regulatory Scrutiny and Legal Risks
- Increased regulatory oversight on drug pricing and procurement could impact operations.
- The “Pfizergate” investigation highlights challenges in politically sensitive environments, with calls for greater clinical trial transparency adding pressure.
Patent Expirations and Generic Competition
- Expiring patents on blockbuster drugs expose Pfizer to revenue losses from generic competitors, particularly in key markets like the U.S. and Europe.
Intensifying Competition
- Pfizer faces strong competition from Moderna, Johnson & Johnson, and AstraZeneca in vaccines, and Merck and Roche in oncology.
- Emerging biotech startups with disruptive technologies further intensify competitive pressures.
Macroeconomic Challenges
- Inflation, rising raw material costs, and supply chain disruptions could increase expenses and affect profitability.
- Currency fluctuations in emerging markets may also pose financial challenges.
Public Perception and Ethical Concerns
Negative sentiment on social media platforms highlights the need for proactive public relations strategies.
Criticism of drug pricing practices and lobbying efforts could harm Pfizer’s reputation and trust among consumers.
Conclusion
Pfizer’s strengths, particularly its global presence, innovative R&D, and financial resilience, establish it as a leader in the pharmaceutical industry. However, the company faces notable weaknesses and threats, including declining COVID-19 revenues, patent expirations, and legal controversies. By leveraging opportunities in emerging markets, mRNA technology, and strategic M&A, Pfizer can mitigate these risks and sustain growth.
Disclaimer: This is not financial or investment advice. You are responsible for any capital-related decisions you make, and only you are accountable for the results.
Source:
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-provides-full-year-2025-guidance-and-reaffirms-full
https://s28.q4cdn.com/781576035/files/doc_events/2024/Dec/17/FINAL-Full-Year-2025-Guidance-Charts.pdf
https://www.biopharmadive.com/news/pfizer-2025-guidance-forecast-wall-street/735725/
https://www.morningstar.com/company-reports/1257606-pfizer-2025-guidance-largely-reassuring-on-covid-stability-and-cost-saving-execution
https://www.bamsec.com/companies/78003/pfizer-inc
https://www.politico.eu/article/pfizergate-covid-vaccine-scandal-european-prosecutors-eu-commission/
https://www.investors.com/news/technology/pfizer-stock-ex-employee-found-guilty-in-paxlovid-tied-insider-trading-scheme/
https://www.nasdaq.com/articles/ex-pfizer-employee-convicted-of-insider-trading-on-covid-drug-trial
https://news.bloomberglaw.com/white-collar-and-criminal-law/ex-pfizer-employee-convicted-of-insider-trading-on-covid-pill
https://www.justice.gov/usao-sdny/pr/friend-pfizer-employee-pleads-guilty-insider-trading-based-non-public-drug-trial
https://investors.pfizer.com/Investors/Financials/Annual-Reports/default.aspx
https://finance.yahoo.com/quote/PFE/
https://www.pfizer.com/newsroom/media-assets
